 In a case with huge financial stakes for the Texas hemp industry (and the millions of Texans who enjoy hemp-derived products), the Austin Court of Appeals has upheld a temporary injunction granted by an Austin district court blocking the Department of State Health Services (DSHJS) from implementing a change to the 2021 Schedule of Controlled Substances that arguably limits the scope of legal hemp production more strictly than federal law allows.
In a case with huge financial stakes for the Texas hemp industry (and the millions of Texans who enjoy hemp-derived products), the Austin Court of Appeals has upheld a temporary injunction granted by an Austin district court blocking the Department of State Health Services (DSHJS) from implementing a change to the 2021 Schedule of Controlled Substances that arguably limits the scope of legal hemp production more strictly than federal law allows.
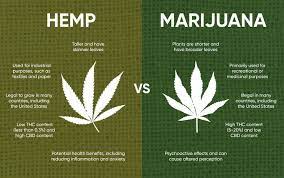
The facts of Texas Department of State Health Services, and Dr. Jennifer A. Shuford, in her Official Capacity as Commissioner of the Texas Department of State Health Services v. Sky Marketing Corp., d/b/a Hometown Hero; Create a Cig Temple, LLC; Darrell Surif; and David Walden (No. 03-21-00571-CV; filed September 28, 2023) are as follows. As you may recall, the 2018 Farm Bill enacted by Congress changed the definition of “marihuana” for purposes of federal schedules of controlled substances to exclude “hemp.” The crux of this change was to continue federal regulation only of tetrahydrocannabinols in hemp where they exceed 0.3% of delta-9 TCH by dry weight. Anything less than that can be legally grown, which is a boon to the farm industry. The Legislature followed up in 2019 by taking the shackles off hemp and hemp-derived products as defined by federal law, and the Department of State Health Services duly amended the 2019 schedule of controlled substances to reflect the changes in state and federal law. After the USDA approved a state plan for the manufacture, distribution, and sale of consumable hemp products, the Department of Health and Human Services adopted rules and procedures to implement the plan.
In 2020 the commissioner of the DSHS, acting under her authority pursuant to the Texas Controlled Substances Act (TCSA), filed an objection to the Drug Enforcement Administration’s Interim Final Rule (IFR), which codified the 2018 Farm Bill changes. The commissioner based her objections to the IFR on its definitions of “tetrahydrocannabinols” and “marihuana extract,” which she argues “allow for the presence or addition of tetrahydrocannabinols aside from the presence of [delta-9 THC],” specifically “delta-8 THC derived or extracted from hemp or CBD.” After a brief public hearing, the commissioner published her decision rejecting the DEA’s definitions. In March 2021, she published the 2021 Schedule of Controlled Substances using the Texas Agriculture Code definitions of those terms, not those contained in the federal statute. The upshot is that all forms of THC, including Delta-8 in any concentration and Delta-9 exceeding 0.3% remain listed as Schedule I controlled substances.
Distributors and sellers of THC products immediately sued the state seeking temporary and permanent injunctions and declaratory relief. They alleged that the commissioner’s amendments were ultra vires because they were not adopted in compliance with the TCSA and were invalid rules under the APA. The state filed a plea to the jurisdiction asserting lack of standing and sovereign immunity. Following a hearing, the trial court denied the plea and granted plaintiff’s request for a temporary injunction. The state appealed.
In an opinion by Justice Theofanis, joined by Chief Justice Byrne and Justice Triana, the court of appeals affirmed. First, the state argued that plaintiffs lacked standing because DSHS, though it publishes the schedules, does not enforce them. The court disagreed and determined that plaintiffs made the requisite pleadings and evidentiary showing that their alleged injuries were both concrete and particularized and “fairly traceable” to the commissioner’s actions. It found further that the alleged injuries could be redressed by an injunction blocking the commissioner’s amendments from going into effect. Plaintiffs thus had standing, and the trial court did not abuse its discretion in denying the state’s plea to the jurisidiction on this ground.
Turning to the ultra vires claim, the court focused on whether the commissioner exceeded her authority under § 481.034, Health & Safety Code (the TCSA). This section sets out the requirements that the commissioner must follow before adding or deleting a substance from the schedules of controlled substances. Plaintiffs allege that the commissioner violated the statute’s prohibition on adding a substance that the legislature had specifically deleted, to wit, delta-8 THC products. They argue further that the commissioner made the changes without complying with the public hearing and executive commissioner approval requirements in § 481.034(b). The state asserted that she acted under an exception to the rule in response to the IFR, but the court rejected this argument, holding that the exception only applies “when she is modifying the schedules to conform them with federal modifications,” not when she is modifying them because she objects to such modifications. The trial court did not abuse its discretion as to the ultra vires claim.
As to the APA claim, the court analyzed whether DSHS’s statement on its website about Delta-8 regulation and modification of the 2021 schedule constituted a “rule” subject to the APA’s rulemaking procedures, and if it is, “whether the rule or its threatened application interferes with or impairs appellees’ legal rights or privileges” (citation omitted). Here the court had no difficulty finding that department’s statement “prescribes law, is generally applicable to the public at large, does not pertain to the Department’s internal management, and impacts personal rights, including appellees’ alleged rights” (citations omitted). Once again, the trial court did not abuse its discretion in denying the state’s plea to the jurisdiction as to the APA claim.
The state’s final issue challenged the temporary injunction itself. The court concluded that “appellees pleaded valid claims against the Department and Commissioner and, based on our review of the evidence as stated above, we conclude the appellees presented some evidence of a probable right to relief” (citations omitted). It found further that appellees presented evidence that the state’s action caused “probable, imminent, and irreparable injury” because it deprived them of a substantial part of their businesses and blocked prior access to Delta-8 products for medical treatment (it should be noted that the director of government and public affairs for the Department of Texas Veterans of Foreign Wars testified on behalf of 68,000 veterans and their families against DSHS and the commissioner). Finally, the court found that the temporary injunction did not change the status quo in place when the commissioner made the schedule changes, that the harm to appellees outweighed any potential harm to DSHS, and that granting the TI benefited the public interest.
All eyes will now turn to SCOTX, which will have to figure out a way to navigate what has become a highly politicized social issue (like just about everything else, we fear). Unfortunately, there will no doubt be more to come.